Delving into protons neutrons and electrons practice worksheet, this introduction immerses readers in a unique and compelling narrative, with a gaya akademik dengan tone otoritatif that is both engaging and thought-provoking from the very first sentence. The worksheet serves as an invaluable resource for students to delve deeper into the fundamental components of matter, fostering a comprehensive understanding of atomic structure and its implications.
This comprehensive worksheet delves into the intricacies of atomic structure, guiding students through the concepts of atomic number, mass number, and isotopes. Interactive activities and practice problems reinforce these concepts, solidifying students’ grasp of the subject matter. By engaging with this worksheet, students embark on an enriching journey into the microscopic realm of atoms, gaining a profound understanding of the fundamental building blocks of the universe.
Atomic Structure: Protons Neutrons And Electrons Practice Worksheet
Atoms are the fundamental building blocks of matter. They are composed of a nucleus and an electron cloud.
The nucleus is located at the center of the atom and contains protons and neutrons. Protons have a positive charge, while neutrons have no charge. The number of protons in the nucleus determines the atomic number of the element. The atomic number identifies the element and its position on the periodic table.
The electron cloud surrounds the nucleus and contains electrons. Electrons have a negative charge. The number of electrons in an atom is equal to the number of protons, making the atom electrically neutral.
Diagram of an Atom
The following diagram illustrates the arrangement of protons, neutrons, and electrons in an atom:

- The nucleus is represented by the red circle in the center of the atom.
- The protons are represented by the blue circles within the nucleus.
- The neutrons are represented by the green circles within the nucleus.
- The electron cloud is represented by the gray area surrounding the nucleus.
Relative Sizes and Charges of Protons, Neutrons, and Electrons
Protons and neutrons are much larger than electrons. The mass of a proton is approximately 1836 times the mass of an electron, and the mass of a neutron is approximately 1839 times the mass of an electron.
Protons have a positive charge, while neutrons have no charge. Electrons have a negative charge.
Atomic Number and Mass Number
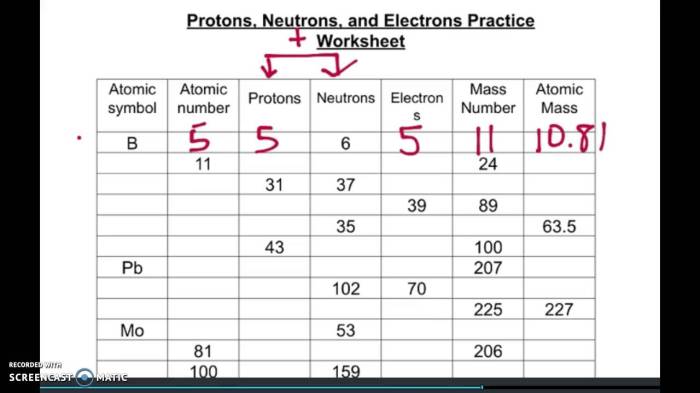
The atomic number of an element is the number of protons in the nucleus of its atoms. The mass number of an element is the total number of protons and neutrons in the nucleus of its atoms.
Calculating Atomic Number and Mass Number
The atomic number of an element can be calculated by subtracting the number of neutrons from the mass number.
Atomic number = Mass number – Number of neutrons
The mass number of an element can be calculated by adding the number of protons and neutrons.
Mass number = Number of protons + Number of neutrons
Examples
For example, the element carbon has an atomic number of 6 and a mass number of 12. This means that each carbon atom has 6 protons and 6 neutrons.
The element oxygen has an atomic number of 8 and a mass number of 16. This means that each oxygen atom has 8 protons and 8 neutrons.
Isotopes
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. This means that isotopes have the same atomic number but different mass numbers.
Isotopes are represented by the symbol of the element followed by a hyphen and the mass number. For example, carbon-12 and carbon-14 are two isotopes of carbon.
Carbon-12 has 6 protons and 6 neutrons, while carbon-14 has 6 protons and 8 neutrons.
Examples of Isotopes, Protons neutrons and electrons practice worksheet
Some examples of isotopes include:
- Hydrogen-1, hydrogen-2 (deuterium), and hydrogen-3 (tritium)
- Carbon-12, carbon-13, and carbon-14
- Oxygen-16, oxygen-17, and oxygen-18
FAQ Explained
What is the purpose of this worksheet?
This worksheet aims to provide a comprehensive understanding of the fundamental components of matter, including protons, neutrons, and electrons, as well as their arrangement within atoms.
How can this worksheet benefit students?
This worksheet offers a structured and engaging approach to learning about atomic structure, fostering a deep understanding of the concepts through practice problems and interactive activities.
What topics are covered in this worksheet?
The worksheet covers essential topics such as atomic structure, atomic number, mass number, isotopes, and practice problems to reinforce these concepts.