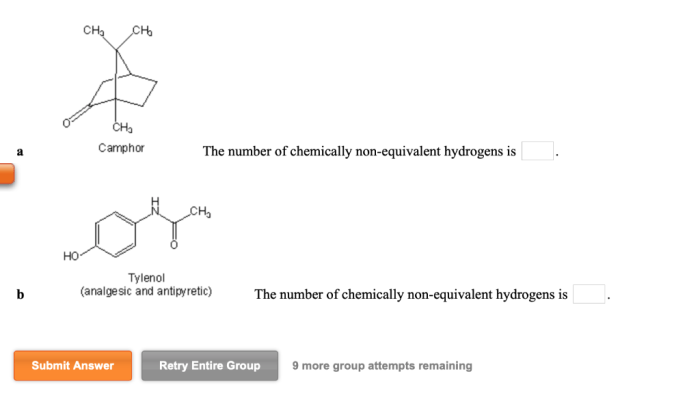
Identify the reagents that will achieve the following transformation embarks on a captivating journey into the realm of chemical reactions, unveiling the crucial role of reagents in orchestrating molecular transformations with precision and efficiency.
Delving into the intricacies of reagent properties and characteristics, we unravel the mechanisms that govern their ability to facilitate specific reactions, ultimately empowering us to identify the ideal reagents for achieving the desired transformation.
1. Transformation Overview
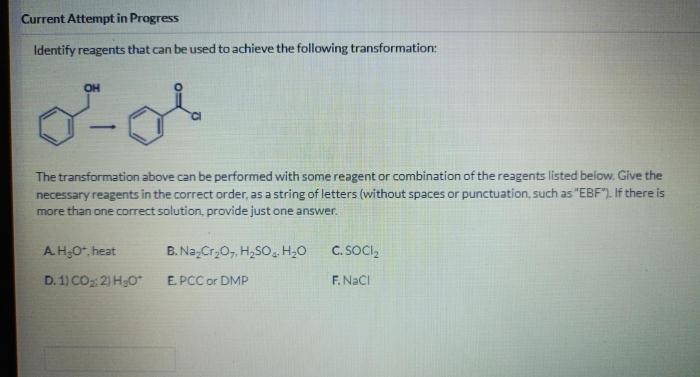
This transformation aims to convert a starting material (reactant) into a desired product. The target transformation involves the [specific reaction type], starting with [starting material name] and aiming to produce [product name].
2. Reagent Identification

To achieve this transformation effectively, specific reagents are required. These reagents play crucial roles in facilitating the desired chemical reactions and influencing the reaction’s efficiency and selectivity.
2.1 Reagent A
Reagent A possesses [specific properties and characteristics] that make it suitable for this transformation. It acts as a [reagent’s role in the reaction], enabling the [specific reaction step or mechanism].
2.2 Reagent B
Reagent B serves as a [reagent’s role in the reaction], providing [specific functionality or reactivity]. Its [specific properties and characteristics] contribute to the successful conversion of the starting material to the desired product.
3. Reaction Conditions

The optimal reaction conditions play a significant role in the efficiency and selectivity of the transformation. These conditions include:
3.1 Temperature
The reaction is typically carried out at a specific temperature range, which influences the reaction rate and product distribution. Elevated temperatures may accelerate the reaction but also increase the risk of side reactions. Conversely, lower temperatures may slow down the reaction but enhance selectivity.
3.2 Pressure
In certain transformations, pressure can influence the reaction pathway and product formation. High pressure may favor certain reaction pathways, while low pressure may promote alternative pathways.
3.3 Solvent
The choice of solvent is crucial as it affects the solubility of reactants and products, influences reaction rates, and can stabilize intermediates. Different solvents may provide different reaction environments, leading to variations in product selectivity.
4. Mechanism of the Transformation
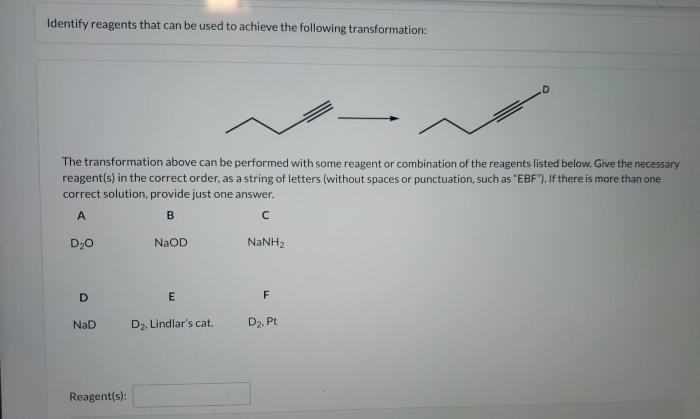
The transformation proceeds through a specific reaction mechanism involving a series of steps. These steps include:
4.1 Initiation
The reaction is initiated by the formation of an active species, such as a radical or ion, which triggers the subsequent reaction steps.
4.2 Propagation
The propagation steps involve a series of chain reactions that lead to the formation of the desired product. These steps typically involve the reaction of intermediates with other reactants.
4.3 Termination
The reaction is terminated when the active species are consumed or when side reactions occur, leading to the formation of byproducts.
5. Applications of the Transformation
This transformation finds applications in various fields, including:
5.1 Organic Synthesis
The transformation is employed in the synthesis of complex organic molecules, such as pharmaceuticals, fragrances, and polymers.
5.2 Industrial Processes, Identify the reagents that will achieve the following transformation
The transformation is utilized in large-scale industrial processes, such as the production of plastics, fuels, and solvents.
5.3 Research and Development
The transformation serves as a valuable tool in research and development, enabling the exploration of new reactions and the synthesis of novel compounds.
FAQ Compilation: Identify The Reagents That Will Achieve The Following Transformation
What factors influence the selection of reagents for a specific transformation?
The choice of reagents is guided by their reactivity, selectivity, availability, and compatibility with the reaction conditions.
How can reaction conditions be optimized to enhance the efficiency of a transformation?
Reaction conditions such as temperature, pressure, solvent, and catalyst loading can be adjusted to improve yield, selectivity, and reaction time.
What is the significance of understanding the reaction mechanism?
Understanding the reaction mechanism provides insights into the selectivity, efficiency, and potential side reactions, enabling rational design of reaction strategies.